CD137 is a member of the tumor necrosis factor (TNF) receptor superfamily. Also referred to as the tumor necrosis factor receptor superfamily member 9 (TNFRSF9) and 4-1BB, CD137 is induced by lymphocyte activation (ILA), and expressed on the surface of activated T cells and NK cells. When Natural ligand CD137L or an agonistic antibody binding to CD137 is activated via NF-kB and MAPK pathways via the adaptor molecule TRAF-2/TRAF-1, T-cell co-stimulation is increased, inducing cell proliferation, cytokine expression, and bactericidal activity, as well as supporting T-cell effector function. CD137 also stimulates the proliferation of NK cells and the production of IFN-γ[2], expressing and mediating the activation of DC by promoting the production of cytokines and the upregulation of B7[3]. Furthermore, CD137 can protect T-cells from activation-induced cell death (AICD) [1], demonstrating that dual and multiple antibodies targeting CD137 show great potential in anti-cancer treatments.
In addition, CD137 is expressed in the blood vessel wall of tumor tissues, and CD137L is expressed on the cell surface of numerous human tumor tissues, which illustrates the importance of CD137 and CD137L in TME.
Development Strategy
GemPharmatech has successfully developed several CD137 humanized mouse models, including single-humanized mice BALB/c-hCD137 and C57BL/6-hCD137, double-humanized mice BALB//c-hPD1/hCD137 and C57BL/6-hPD1/hCD137, and triple-humanized mice BALB/c-hPD1/hPDL1/hCD137 by replacing parts of the CD137 gene-encoding extracellular domain of BALB/c and C57BL/6 mice with corresponding humanized gene sequences. As this process maintains the integrity of the murine sequence in the cytoplasmic domain, the CD137 humanized mouse models can be used for efficacy evaluation and safety evaluation of human CD137 agonists.
1. BALB/c-hCD137: Urelumab Drug Efficacy Test
Testing the anti-tumor effect of human CD137 antibody-Urelumab in BALB/c-hCD137 mouse models after subcutaneous transplantation with CT26.WT tumor cells.
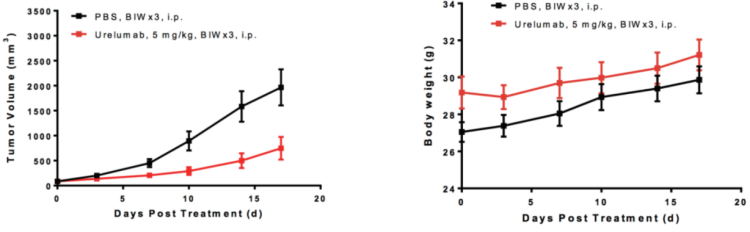
Left Figure: Mouse tumor growth curve Right Figure: Mouse body weight curve
Colon cancer cells CT26.WT in the logarithmic growth phase were subcutaneously transplanted into 5-8 weeks old (WO) BALB/c-hCD137 humanized mice. When tumors reached an average volume of about 100 mm3, the mice were randomized into either the vehicle (Control) Group or the Urelumab Group (n = 6) and treated with the corresponding drugs. The drugs are administered twice a week for a total of 6 doses. The data were presented as Mean ± SEM.
Results: Anti-human CD137 antibody Urelumab significantly inhibited tumor growth (TGI = 64.26%).
These results demonstrate that the BALB/c-hCD137 mouse is an ideal animal model for evaluating the efficacy of human CD137 antibodies.
2. C57BL/6-hCD137: Urelumab and Utomilumab Drug Efficacy Test
Analyzing the anti-tumor effects of human CD137 antibody-Urelumab and Utomilumab analogs in C57BL/6-hCD137 mouse models after subcutaneous transplantation with MC38 tumor cells.
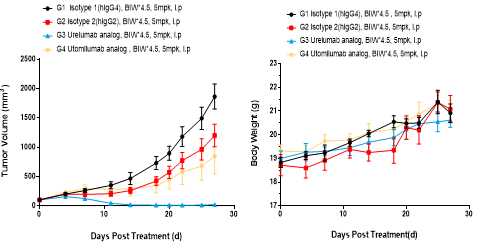
Left Figure: Mouse tumor growth curve Right Figure: Mouse body weight curve
Murine colon cancer cells MC38 were subcutaneously transplanted into C57BL/6-hCD137 humanized mice. When tumors reached an average volume of about 100 mm3, the mice were randomized to either the Control or Treatment Group (n = 6). The data are presented as Mean ± SEM.
Results: The anti-human CD137 antibody Urelumab had a significant inhibitory effect on tumor growth (TGI = 100%), while the anti-human CD137 antibody Utolumab showed only partial tumor inhibitory effect (TGI = 47.67%); the efficacy results were in agreement with the clinical outcomes.
These results demonstrate the C57BL/6-hCD137 mouse as a powerful instrument for evaluating the in vivo efficacy of human CD137 antibodies.
3. BALB/c-hPD1/hPDL1/hCD137: Urelumab and anti-PD1 drug efficacy test
Analyzing the antitumor efficacy of anti-human CD137 antibody-Urelumab and anti-hPD1 antibody in BALB/c-hPD1/hPDL1/hCD137 mouse models following subcutaneous transplantation with CT26-hPDL1(Tg)-mPDL1(KO) tumor cells.
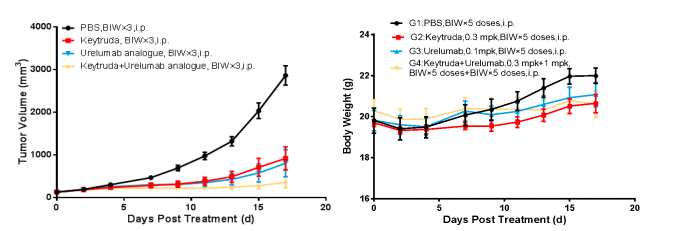
Left Figure: Mouse tumor growth curve Right Figure: Mouse body weight curve
In vivo efficacy test in a model of BALB/c-hPD1/hPDL1/hCD137 mice subcutaneously inoculated with CT26-hPDL1(Tg)-mPDL1(KO). Colon cancer cells CT26-hPDL1(Tg)-mPDL1(KO) in the logarithmic growth phase were subcutaneously inoculated into 6-8 w BALB/c-hPD1/hPDL1/hCD137 humanized mice. When tumors reached an average volume of 100 mm3, the mice were randomized to PBS Group, Keytruda Group, Urelumab Analog Group, or Keytruda + Urelumab Analog Group (n = 8) and treated with the corresponding drugs. The drugs were administered twice a week for a total of 5 doses. The data are presented as Mean ± SEM.
Results: Tumor growth inhibition (TGI) was 69.68% and 72.21%, respectively, in the Keytruda Group and the Urelumab Analog Monotherapy Group; 87.22% in the Keytruda + Urelumab Analog Group. TGI was significant in all treatment groups but particularly so in the combination therapy Group.
BALB/c-hPD1/hPDL1/hCD137 mice can be used to evaluate the antitumor effect of humanized CD137 antibodies, PD-1/PD-L1 antibodies, and their combination therapy.
4. C57BL/6-hPD1/hCD137: Anti-CD137 Ab and Keytruday
Testing the antitumor effect of anti-human CD137 antibody and anti-hPD1 antibody KEYTRUDA® (Pembrolizumab) after subcutaneous MC38 tumor cell line transplantation in C57BL/6-hPD1/hCD137 mouse models.
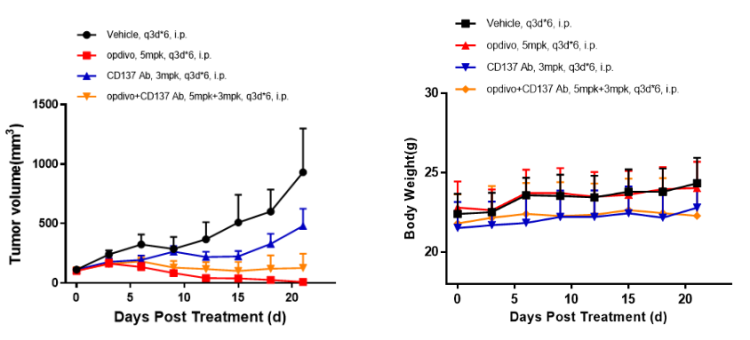
Left Figure: Mouse tumor growth curve Right Figure: Mouse body weight curve
Colon cancer cells MC38 in the logarithmic growth phase were subcutaneously transplanted into 6-8 WO C57BL/6-hPD1/hCD137 humanized mice to assess the in vivo effectiveness of anti-human CD137 antibodies and anti-human PD1 antibodies. When tumors reached an average volume of 100 mm3, the mice were randomized to vehicle Group (n = 5), CD137 Ab 3 mg/kg Group (n = 5), Opdivo 5 mg/kg Group (n = 4) and CD137 Ab 3 mg/kg + Opdivo 5 mg/kg Group (combination therapy Group, n = 5). The drugs were administered every three days for a total of 6 doses. The data are presented as Mean ± SEM.
Results: Significant tumor growth inhibition was observed in the CD137 Ab + Opdivo combination therapy Group (TGI = 86.2%) and the Opdivo 5 mg/kg Group (TGI = 98.8%), while partial tumor growth inhibition was observed in the CD137 Ab 3 mg/kg Group (TGI = 48.3%) (Left Figure). No significant change in the body weight of mice was observed in any of the four groups (Right Figure).
The C57BL/6-hPD1/hCD137 mouse is an ideal animal model for evaluating the efficacy of anti-human CD137 antibody + anti-human PD1 antibody combination therapies.
References:
[1] Cannons JL, et al. 4-1BB ligand induces cell division, sustains survival, and enhances effector function of CD4 and CD8 T cells with similar efficacy. J.Immunol. 2001; 167:1313‒1324.
[2] Wilcox RA, Tamada K, Strome SE, Chen L. Signaling through NK cell-associated CD137 promotes both helper function for CD8+ cytolytic T cells and responsiveness to IL-2 but not cytolytic activity. J. Immunol. 2002; 169:4230‒4236.
[3] Wilcox RA, et al. Cutting edge: expression of functional CD137 receptor by dendritic cells. J. Immunol. 2002;168:4262‒4267.

