Lymphocyte activation protein 3, or LAG3, is expressed on activated T-lymphocytes, B-lymphocytes, natural killer cells, and plasmacytoid dendritic cells [1, 2]. The membrane protein encoded by the LAG3 gene contains four immunoglobulin superfamily domains, which are similar to CD4 in structure and chromosomal location [3], and it binds to MHC II (central histocompatibility complex Class II molecule) [4] with higher affinity and negatively regulates the function of T-cells [5, 6]. Research has demonstrated that the knockout of the LAG3 gene or the inhibition of LAG3 can stimulate the aggregation of antigen-specific CD8+ T lymphocytes, as well as the development of effector functions in organs or tumors[7], making the blockage of LAG3 a great potential tumor therapy.
Development Strategy
GemPharmatech has successfully developed LAG3 humanized mouse models, including single-humanized BALB/c-hLAG3 and C57BL/6-hLAG3 mice, as well as double-humanized BALB/c-hPD1/hLAG3 and C57BL/6-hPD1/hLAG3 mice, by replacing the extracellular domain part of the LAG3 gene of BALB/c and C57BL/6 mice with a corresponding humanized gene sequence. LAG3 humanized mouse models are ideal animal models for efficacy evaluation and safety evaluation of human LAG3 inhibitors.
1. C57BL/6-hLAG3: anti-mPD1 and anti-hLAG3 Drug Efficacy Test
The tumor inhibitory efficacy of anti-human LAG3 antibodies was tested in a model of C57BL/6-hLAG3 mice subcutaneously inoculated with MC38 tumor cell line.
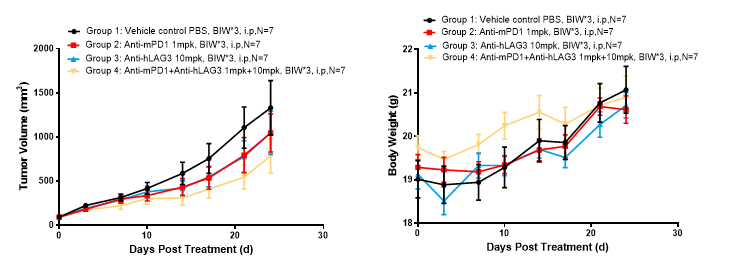
Left Figure: Mouse tumor growth curve Right Figure: Mouse body weight curve
In vivo efficacy test in a model of C57BL/6-hLAG3 mice subcutaneously inoculated with MC38. Colon cancer cells. MC38 were cultured to logarithmic growth phase and inoculated subcutaneously into 6-8 w C57BL/6-hLAG3 mice (LAG3 humanized mice). When tumors reached an average volume of about 90 mm3, the mice were randomized to Vehicle (Control) Group, Anti-mPD1 monotherapy Group, Anti-hLAG3 monotherapy Group, Anti-mPD1 + Anti-h LAG3 combination therapy Group (n = 7) and treated with the corresponding drugs. The drugs were administered twice a week for a total of 6 doses. The data are presented as Mean ± SEM.
Results: Tumor growth inhibition (TGI) was observed in Anti-mPD1 monotherapy Group (TGI = 19.13%), Anti-hLAG3 monotherapy Group (TGI = 21.13%), significant tumor inhibition was observed in the combination therapy Group of Anti-mPD1 + Anti-hLAG3 (TGI = 40.69%) (Left Figure), and these observations were consistent with the body weight trending of mice (Right Figure).
These results demonstrate that humanized LAG3 mouse mice are a powerful instrument for the evaluation of in vivo pharmacodynamics of anti-hLAG3 antibodies.
2. BALB/c-hPD1/hLAG3:Keytruda and anti-hLAG3 Efficacy Test
The antitumor effect of the anti-human PD1 antibody KEYTRUDA® (Pembrolizumab) and Anti-hLAG3 was tested in BALB/c-hPD1/hLAG3 mice subcutaneously inoculated with CT26.WT tumor cell line.
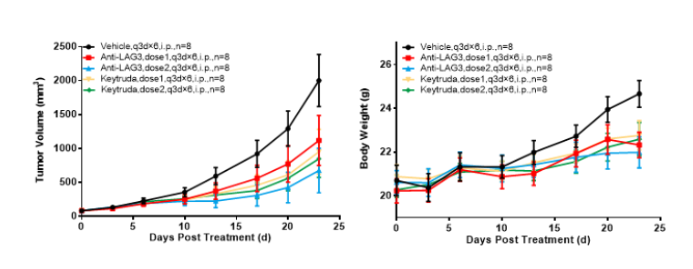
Left Figure: Mouse tumor growth curve Right Figure: Mouse body weight curve
Colon cancer cells CT26.WT in the logarithmic growth phase were subcutaneously transplanted into BALB/c-hPD1/hLAG3 mice. When tumors reached an average volume of 100 mm3, the mice were randomized to Vehicle Group, Anti-LAG3 treatment Group, and Keytruda treatment Group (n = 8). The drugs were administered every three days for a total of six doses. The data are presented as Mean ± SEM.
Results: Tumor growth inhibition was observed in Anti-LAG3 Group (dose1, TGI = 46.83%) and Anti-LAG3 Group (dose2, TGI = 70.29%), the tumor inhibitory effect was better with Anti-LAG3 at dose2. The TGI was 58.00% and 60.52%, respectively, in Keytruda (dose1) and Keytruda (dose2) Groups, suggesting that Keytruda had similar (significant) tumor inhibitory effect at the two dose levels.
These results showcase that the PD1and LAG3 double-humanized mouse is an ideal animal model for evaluating the in vivo efficacy of anti-hPD1 + anti-hLAG3 antibody combination therapy.
3. C57BL/6-hPD1/hLAG3: anti-hPD1 and anti-hLAG3 Efficacy Test
The tumor inhibitory efficacy of anti-human PD1 and LAG3 antibodies was tested in a model of C57BL/6-hPD1/hLAG3 mice subcutaneously inoculated with MC38 tumor cell line.
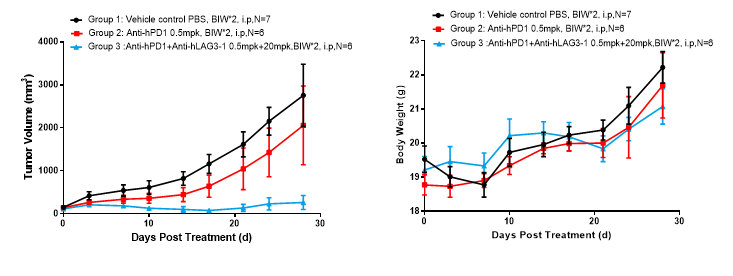
Left Figure: Mouse tumor growth curve Right Figure: Mouse body weight curve
Colon cancer cells MC38 in the logarithmic growth phase were subcutaneously inoculated into 6-8 w C57BL/6-hPD1/hLAG3 mice (PD1 and LAG3 double-humanized mice). When tumors reached an average volume of about 80-120 mm3, the mice were randomized to Vehicle Group (n = 7), Anti-hPD1 monotherapy Group (n = 6), Anti-hPD1 and Anti-hLAG3 combination therapy Group (n = 6) and treated with the corresponding drugs. The drugs were administered twice a week for a total of four doses. The data are presented as Mean ± SEM.
Results: Minimal tumor inhibition was observed in the Anti-hPD1 monotherapy Group (TGI = 25.64%), and significant tumor inhibition was observed in the Anti-hPD1 + Anti-hLAG3 combination therapy Group (TGI = 89.29%) (Left Figure), and these observations were consistent with the body weight trending of mice (Right Figure).
These results demonstrate that the PD1/LAG3 double-humanized mouse is a powerful instrument for evaluating the in vivo efficacy of anti-hPD1 + anti-hLAG3 antibody combination therapy.
References:
[1] Sierro, Sophie, Pedro Romero, and Daniel E. Speiser. "The CD4-like molecule LAG-3, biology and therapeutic applications." Expert opinion on therapeutic targets 15.1 (2011): 91-101.
[2] Goldberg, Monica V., and Charles G. Drake. "LAG-3 in cancer immunotherapy." Cancer Immunology and Immunotherapy. Springer, Berlin, Heidelberg, 2010.269-278.
[3] Triebel, Frederic, et al. "LAG-3, a novel lymphocyte activation gene closely related to CD4." Journal of Experimental Medicine 171.5 (1990): 1393-1405.
[4] Huard, Bertrand, et al. "CD4/major histocompatibility complex class II interaction analyzed with CD4‐and lymphocyte activation gene‐3 (LAG‐3)‐Ig fusion proteins." European journal of immunology 25.9 (1995): 2718-2721.

