Acute myelogenous leukemia (AML), which accounts for nearly 70% of adult leukemias, is one of the most challenging leukemias to cure. FLT3 (FMS-like tyrosine kinase 3), also known as Flk2/CD135, is a type III receptor tyrosine kinase (RTK) that is critical for the survival, proliferation, and differentiation of hematopoietic cells. FLT3 gene mutations are a common genetic variant in AML (approximately 30%) and are associated with a poor clinical prognosis and susceptibility to relapse. Consequently, FLT3 inhibitor research is critical for AML treatment. The construction of the AML-PDX model requires additional human cytokines to help AML leukemia cells escape residual innate immune surveillance in mice in a specific supportive stromal cell milieu and subsequently home and expand in mice. GemPharmatech's NCG-M mouse strain outperforms a conventional, severely immunodeficient mouse without human cytokine assistance in terms of modeling efficiency. AML-PDX models with FLT3 mutations have been developed and are suitable for drug sensitivity testing as well as preclinical efficacy evaluation of various AML therapies.
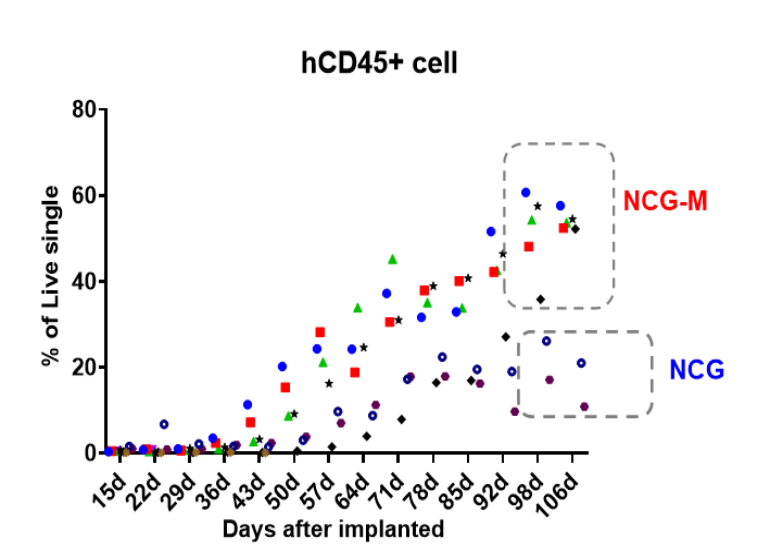
Figure 1. The hCD45+ cells percentage in peripheral blood increased gradually after AML-PDX modeling. The NCG-M mouse strain outperformed the NCG strain in terms of modeling efficiency.
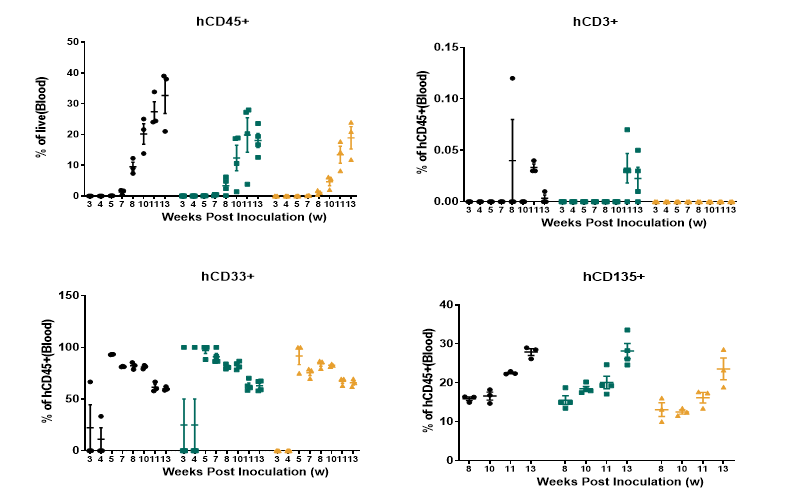
Figure 2. Characteristic markers such as hCD45+, hCD33+, and hCD135+ (FLT3) were identified in the peripheral blood of mice at various stages after the AML-PDX models were passaged. Lower inoculum doses successfully amplified AML-PDX models in the NCG-M strain, and the efficacy and quality of modeling were dose-dependent.