Programmed cell death 1 ligand 1 (PDL1), also known as cluster of differentiation 274 (CD274) or B7 homolog 1 (B7-H1), is a type I transmembrane protein of 40 kDa encoded by the CD274 gene. Several studies have confirmed that the expression of PDL1 on the surface of tumor cells is significantly increased in the tumor microenvironment, and that it binds to PD1 on the surface of activated T cells, transmitting suppressive regulatory signals. This, in turn, causes the apoptosis or immune disability of tumor antigen-specific T cells and suppresses the immune response. The blockage of the PD1/PDL1 signaling pathway has become a classic method for tumor immunotherapy [1-2].
Development Strategy
GemPharmatech developed and utilizes PDL1 humanized mouse models, including single-target model BALB/c-hPDL1 mice and C57BL/6-hPDL1 mice, as well as double-target model PDL1BALB/c-hPD1/hPDL1 mice and C57BL/6-hPD1/hPDL1 mice, by replacing the extracellular region of BALB/c and C57BL/6 murine PDL1 with the corresponding humanized segments without impairing the integrity of the intracellular region of murine PDL1. PDL1 humanized mouse models are ideal animal models for efficacy evaluation and safety evaluation of human PDL1 inhibitors.
1. BALB/c-hPDL1: TECENTRIQ® Drug Efficacy Test
BALB/c-hPDL1 mice were inoculated subcutaneously with CT26-hPDL1(Tg)-mPDL1(KO) tumor cell line to investigate the tumor inhibition effect of TECENTRIQ® (Atezolizumab), an anti-human PDL1 antibody.
TECENTRIQ® (Atezolizumab): An anti-human PD-L1 antibody anti-tumor drug marketed by Roche.
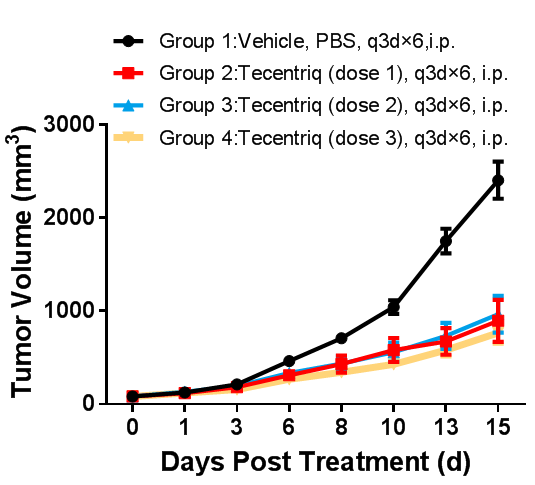
In vivo efficacy study in BALB/c-hPDL1 mouse model inoculated subcutaneously with CT26-hPDL1(Tg)-mPDL1(KO) Colon cancer CT26-hPDL1(Tg)-mPDL1(KO) cells expressing humanized PDL1 protein were cultured to log-phase growth, then were subcutaneously inoculated to BALB/c-hPDL1 humanized mice at the age of 6 - 8 weeks. When the mean tumor volume reached approximately 100 mm3, mice were randomly divided into Vehicle Group or Tecentriq Groups (n = 10). The drugs were administered every three days for a total of 6 doses. The data are presented as Mean ± SEM.
Results: Tumor growth inhibition (TGI) results were 59.62%, 67.73%, and 66.99%, respectively, in Tecentriq 5 mpk, Tecentriq 10 mpk, and Tecentriq 15 mpk Groups, indicating that Tecentriq had a significant inhibitory effect on tumor growth in a dose-dependent manner.
2. C57BL/6-hPDL1: TECENTRIQ® Drug Efficacy Test
Anti-human PDL1 antibody TECENTRIQ® (Atezolizumab) was administered to B6-hPDL1 mice subcutaneously injected with the MC38-hPDL1 tumor cell line to examine the tumor-inhibiting efficacy.
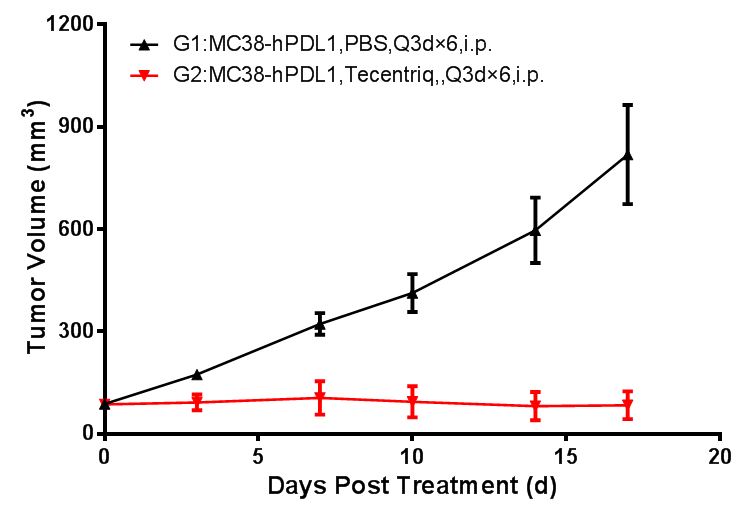
Mouse tumor growth curve
Colon cancer cells MC38-hPDL1(Tg)-mPDL1(KO) expressing humanized PDL1 protein were cultured to log-phase growth, then were subcutaneously inoculated to B6-hPDL1 humanized mice at the age of 6 - 8 weeks. When the mean tumor volume reached approximately 100 mm3, mice were randomly divided into Vehicle Group or Tecentriq Groups (n = 8) for treatment with the corresponding drug. The drugs were administered every three days for a total of 6 doses. The data are presented as Mean ± SEM.
Results: Tecentriq inhibited tumor growth (TGI=87%).
The results demonstrated that the C57BL/6-/hPDL1 mouse is an ideal model for evaluating the anti-tumor effects of anti-human PD1 antibodies, anti-human PDL1 antibodies, and their combinations.
3. C57BL/6-hPD1/hPDL1: KEYTRUDA® and TECENTRIQ® Drug Efficacy Test
The tumor inhibitory efficacy of anti-human PD1 antibody KEYTRUDA® (Pembrolizumab) and anti-human PD-L1 antibody TECENTRIQ® (Atezolizumab) was tested in B6-hPD1/hPDL1 mice subcutaneously inoculated with MC38-hPDL1(Tg)-mPDL1(KO) tumor cell line.
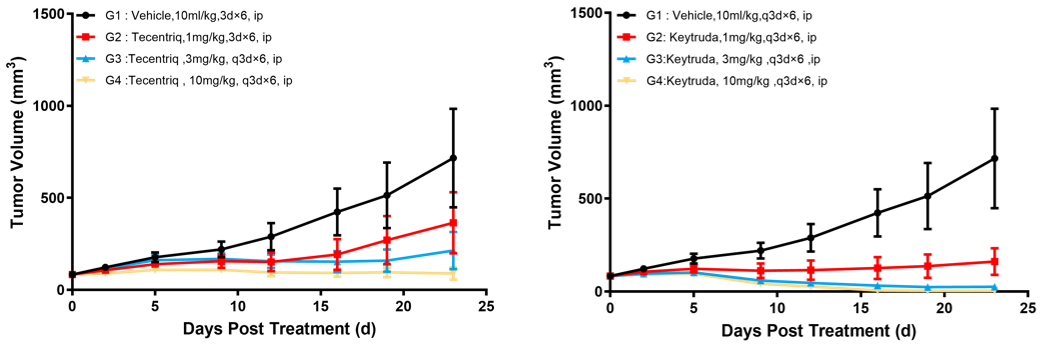
Left figure: Anti-tumor efficacy of Tecentriq. Right figure: Anti-tumor efficacy of Keytruda.
Murine colon cancer cells MC38-hPDL1(Tg)-mPDL1(KO) at log-phase growth were subcutaneously inoculated to C57BL/6-hPD1/hPDL1 mice at the age of 6–8 weeks. When the mean tumor volume reached approximately 100 mm3, mice were randomly divided into Tecentriq 1 mpk, Tecentriq 3 mpk, Tecentriq 10 mpk, Keytruda 1 mpk, Keytruda 3 mpk, and Keytruda 10 mpk Groups (n = 8) for treatment with the corresponding drug. The drugs were administered every three days for a total of 6 doses.
Results: Tumor growth inhibition (TGI) results were 48.26%, 67.77%, and 88.52%, respectively, in Tecentriq 1 mpk, Tecentriq 3 mpk, and Tecentriq 10 mpk Groups, and were 79.63%, 96.45%, and 99.52%, respectively, in Keytruda 1 mpk, Keytruda 3 mpk, and Keytruda 10 mpk Groups, indicating that both antibody-based drugs had a significant inhibitory effect on tumor growth in a dose-dependent manner.
The results demonstrated that the C57BL/6-hPD1/hPDL1 mouse is an ideal model for evaluating the anti-tumor effects of anti-human PD1 antibodies, anti-human PDL1 antibodies, and their combinations.
References:
[1] Immunotherapy: Benefit with anti-PD-L1. Nat Rev Clin Oncol. 2017, 14(2):70-71.
[2] Targeting the PD-1 pathway: a promising future for the treatment of melanoma. Arch Dermatol Res. 2014, 306(6):511-9.

