Patient Derived Xenograft (PDX) Mouse Models and Efficacy Services
Immune Checkpoint Inhibitors represent a prominent hot spot in the R&D of anti-tumor drugs, where the efficacy and safety evaluations of immune checkpoint inhibitors require ideal animal models. Though a perfect animal model is expected to have an intact immune system, as immune checkpoint therapies rely on the immune system to achieve their actions, immune checkpoints have different antibody recognition sites due to their varied molecular conservatism across animal species. Therefore, drug efficacy and safety evaluation in animal models with humanized immune checkpoint modules yield a higher clinical conversation rate.
By establishing a series of mouse models with humanized immune-checkpoint genes and independent intellectual property rights, which are developed by replacing the extracellular regions of murine immune checkpoint molecules with the corresponding human sequences, GemPharmatech models are capable of broad recognition and evaluation of antibodies targeting human immune checkpoints while simultaneously preserving transmembrane and intracellular regions of the murine immune checkpoint genes to ensure the intact and accurate intracellular signaling. These humanized mice are ideal models for efficacy and safety evaluation of immune checkpoint inhibitors.
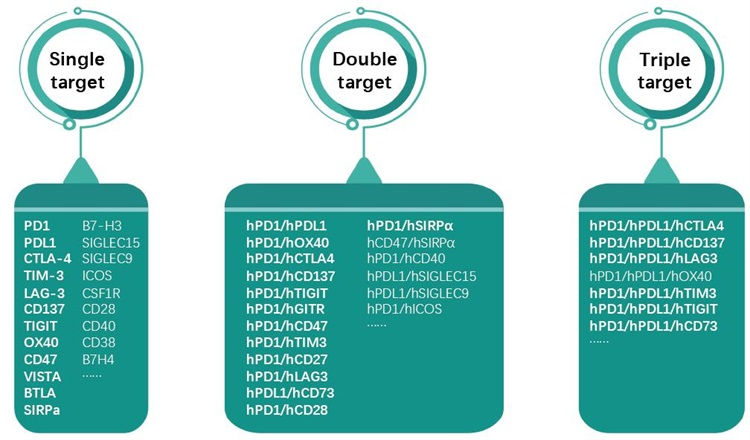
GemPharmatech offers the following 30+ humanized mice with dual genetic backgrounds (BALB/c and C57BL/6):